QUANTUM NUMBERS
1)The Principle Quantum Number "n" : This quantum number was the first one discovered and it was done so by Niels Bohr in 1913. Bohr thought that each electron was in its own unique energy level, which he called a "stationary state," and that each electron would have a unique value of 'n'
:In this idea, Bohr was wrong. It very quickly was discovered that more than one electron could have a given 'n' value. For example, it was eventually discovered that when n=3, eighteen different electrons could have that value.
:Keep in mind that it is the set of four quantum numbers that is important. As you will see, each of the 18 electrons just mention will have its own unique set of n, l, m, and s.
:Finally, there is a rule for what values 'n' can assume. It is:
n = 1, 2, 3, and so on.
: n Will always be a whole number NEVER less than one.
:One point: n does not refer to any particular location in space or any particular shape. It is one component (of four) that will uniquely identify each electron in an atom.
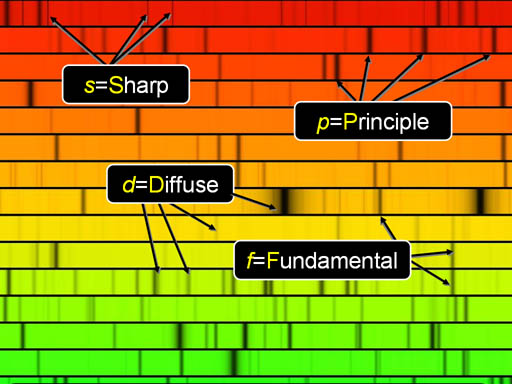
2) The Azimuthal Quantum Number "l" : about 1914-1915, Arnold Sommerfeld realized that Bohr's 'n' was insufficient. In other words, more equations were needed to properly describe how electrons behaved. In fact, Sommerfeld realized that TWO more quantum numbers were needed.
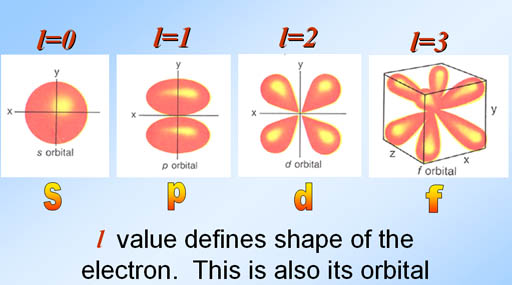 :The rule for selecting the proper values of 'l' is as follows:
:The rule for selecting the proper values of 'l' is as follows:l = 0, 1, 2, . . . , n-1
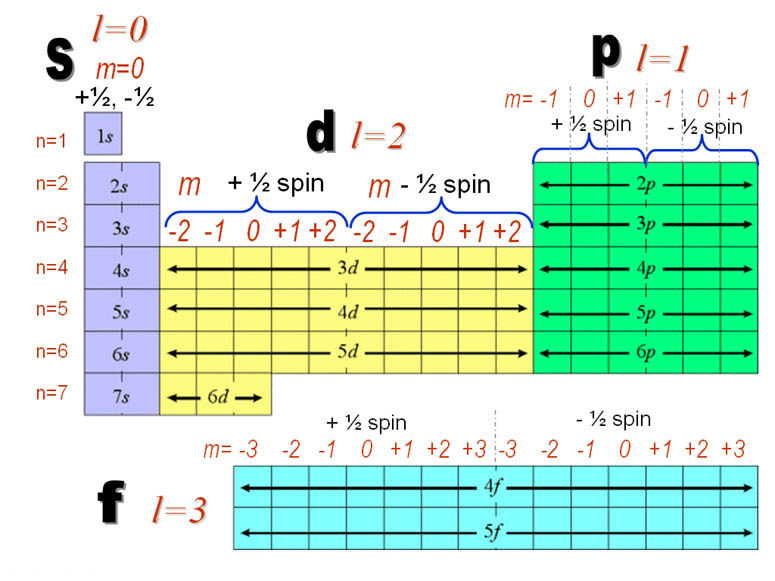
3) Magnetic Quantum Number "ml" :
Specifies the orientation in space of an orbital of a given energy (n) and shape (l). This number divides the subshell into individual orbitals which hold the electrons; there are 2l+1 orbitals in each subshell. Thus thes subshell has only one orbital, the p subshell has three orbitals, and so on.
4) Spin Quantum number "ms" : Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions (sometimes called up and down).
: The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states that no two electrons in the same atom can have identical values for all four of their quantum numbers. What this means is that no more than two electrons can occupy the same orbital, and that two electrons in the same orbital must have opposite spins
: Because an electron spins, it creates a magnetic field, which can be oriented in one of two directions. For two electrons in the same orbital, the spins must be opposite to each other; the spins are said to be paired. These substances are not attracted to magnets and are said to be diamagnetic. Atoms with more electrons that spin in one direction than another contain unpaired electrons. These substances are weakly attracted to magnets and are said to be paramagnetic.


Intolerance is the most socially acceptable form of egotism, for it permits us to assume superiority without personal boasting. See the link below for more info.
ReplyDelete#assume
www.ufgop.org