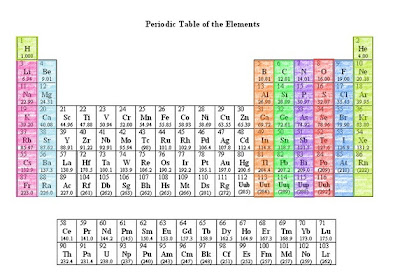
- A group is a vertical column in the Periodic Table of Elements. They are considered the most important method in classifying the different elements.
- Groups are the elements having the same outer electron arrangement. The outer electrons are also called the valence electrons.
- Since they have the same number of valence electrons, the elements in a group shares the same chemical properties.
- The Roman numerals listed ABOVE each group are the usual number of valence electrons.
- These are the groups: IA, IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA
What are Families?
- Groups are also known as Families. The families are the names representing each group.
- There are 9 different families, and these are the:
- Alkali Metals
- Alkaline Earth Metals
- Transition Metals
- Boron
- Carbon
- Nitrogen
- Chalcogens
- Halogens
- Noble Gases
What are Periods?
- Periods are the horizontal rows of elements found in the Periodic Table of Elements. There are 7 periods in the Periodic Table.
- The periods represents the energy level of an atom.
- The number of elements in a period increases as you move down because there are more sub levels per level as the energy level of the atom increases.
- According to the table below, not all the periods have the same number of elements.
| Period Number | Number of Elements |
| 1 | 2 |
| 2 | 8 |
| 3 | 8 |
| 4 | 18 |
| 5 | 18 |
| 6 | 32 |
| 7 | 28 |
What are Valence?
- Valence is also known as valency or valency number, is a measure of the number of chemical bonds formed by the atoms of a given element.
- Valence is the number of electrons needed to fill the outermost shell of an atom.
- For example, when you go across the table from carbon to nitrogen to oxygen, the number of valence electrons increases from 4 to 5 to 6. As we go from fluorine to neon to sodium, the number of valence electrons increases from 7 to 8 and then drops down to 1 when we start the new period.
How to get the element using the periods and groups!
- You can find an element even without the given atomic number just by using the groups and periods. Example: Look for the element that is located on Period 2, Group 4. The element is Carbon because its on the row of period 2 and it is under Group 4.
Try this! Find the element given the period and group number only.
- Period 4, Group 6
- Period 2, Group 2
- Period 5, under the Alkali Metals
- Halogens family, along period 5.
________________________________________________________________
Made by: Group 3
Marj Mendoza
Roxy Trillanes
Isabella Meily
Eula Manibog
Djoseth Macomb

Ayee Wat R De Lists?
ReplyDeleteayyy idk
DeleteAyyy i agree
DeleteAyyyyyyyyyyyyyyyyyyyy
DeleteAyyyyyy
DeleteI'm confused
ReplyDeleteI love this
ReplyDeleteit doesnt love u back
Deleteyes... yes it does
ReplyDeleteturkeys
ReplyDeletehey guys what you doing today
ReplyDeletetayshawn is my baby
ReplyDeletecap
Delete
ReplyDeletewhat the heck
ReplyDeleteI love it really
ReplyDelete