During the nineteenth century, chemists though it logical to arrage the known elements in a table according to their atomic masses. Which such an arrangement, several scientists observed some trends in the chemical and physical properties of successive elements
1829
Green Chemst Johann W. Döbereiner showed that the atomic mass of the element strontium (Sr) lies midway between the atomic masses of calcium (Ca) and barium (Ba), forming what he called a triad. Some years later, he shows that other such triads existed such as halogens chlorine (Cl), bromine (Br), and iodine (I), and the alkali metals lithium (Li), sodium (Na), and potassium (K).
In each triad, the middle element had an atomic mass almost equal to the average atomic mass of the other two elements. However, there was no explainable to be offered to account for this observation at the time.
Other scientists expanded on Döbereiner's suggestions by showing that similar relationships among elements extended further than the triads. Florine (F) was added to the halogens, and magnesium (Mg) to the alkaline earth metals. On the other hand, the elements oxygen (O), sulfur (S), selenium (Se), and tellurium (Te) were classified as a family of elements.
1864
English chemist John Newlands observed that when elements are arranged in increasing atomic masses, there appeared to be a repetition of similar properties for every eight element. Newlands referred to this arrangement as the law of octaves. However, the law of octaves was found to inapplicable for elements after calcium and noble gases were unknown at this time.
 |
| Newland's Law of Octaves |
German chemist Julius Lothar Meyer devised a classification of elements into a table that accounted for the periodic variations in properties. His table included 56 elements.
Russian chemist Dmitri Mendeleev observed that the elements were arranged in increasing atomic masses with similar physic and chemical properties were periodically prepared. By arranging the elements so that those with similar properties were in the same column, he constructed the periodic table. Mendeleev noted that in order for the elements with similar properties to fall under the same column, there had to be gaps in the middle. This led him to believe that the gaps represented new elements to be discovered in the future. Further more, Mendeleev predicted he properties of these yet to be discovered elements, as well as the properties of their compounds.
- Both Mendeleev and Meyer arranged elements in increasing atomic mass.
- Both left vacant spaces where unknown elements should fit.
- Mendeleev is called the "Father of the Modern Periodic Table" because he corrected the atomic masses of beryllium (Be), indium (In), and uranium (U).
- Mendeleev's table was based on the original periodic law, which stated that the physical and chemical properties of elements are period functions of their atomic masses. So if the elements were arranged in increasing atomic masses, those elements with similar properties would appear at regular intervals. His table contained 66 known elements.
Problems arose later when more accurate masses were determined. The periodic law and its classificaltion to increased atomic masses cause several elements to be incorrectly placed in the table, which led to scientists thinking that another propery, aside from atomic mass, must be the basis for the similarity of the properrites of the elements that are close together in the periodic table.
English physicist Henry Moseley observed that the frequencies of X-rays emitted from atoms of elements were correlated with the sizes of their nuclear charges. Moseley called this nuclear charge of an atom atomic number (Z). He published the results of his work on 39 elements, arranging them in increasing atomic numbers. His work was perhaps the most fundamental single step in the development of the periodic table because e was able to solve Mendeleev's irregularities in his periodic table.
Glenn Seaborg co-discovered 10 elements with Edwin McMillan. He moved 14 elements out of the main body to their current locations. He was the only person to have an element named after him while he was still alive (Seaborgium, Sg)
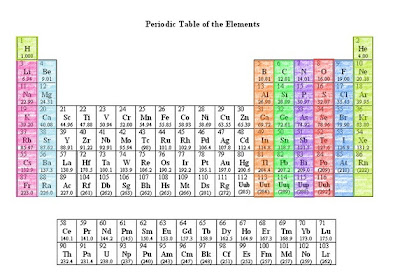
The Modern Periodic Table
The modern version of the periodic table is arranged in increasing atomic number oder. Each square in the periodic table shows the symbol of each element, its atomic number, atomic mass, and other additional information.
As of 2002, the modern periodic table has 114 elements. By 1940, all the natural-occuring elements, which are about 90, had already been discovered. More than 20 elements are artificially made or are naturally-occurring, but is short lived.
The modern periodic law states that the chemical and physical properties of elements are periodic functions of their atomic numbers. Here, periodic function refers to the repetition of the properties of elements a regular intervals.
Features of the Periodic Table
- Most useful to a chemist
- Organizes information about elements
- Elements are arranged in rows by atomic number
- Horizontal rows are Periods.
- Vertical columns are Groups or Families. Elements have similar chemical and physical properties and have the same valence shells (Found on the last energy level).
- Metals
- Metalloids
- Noble gases
- Non-metals
 |
| The Modern Periodic Table of Elements |
Group I
Michaela Panaguiton
Nadine Leano
Ella Canuel
Jenny Gonzales
Published by Michaela Panaguiton